New Projects
All new projects must be reviewed by the IRB prior to the conduct of any research involving human subjects. On this page, you will find directions for submitting a new project to the IRB.
In This Section
- Directions for Submitting a New Project to the IRB
- What Comes Next?
- Example Submissions
- Additional Resources
- Related Topics
Directions for Submitting a New Project to the IRB
Getting Started
Read the UC Davis Investigator Manual
The Investigator Manual outlines the roles and responsibilities for those engaged in human subjects research at UC Davis. All Principal Investigators must read the Investigator Manual before starting their research.
Complete CITI training
All investigators and staff engaged in human subjects research at UC Davis must complete CITI training. CITI training is valid for 3 years and must be renewed prior to expiration.
The specific CITI training courses required for your research depend on the type of research you are conducting. The Required Education Page has a link to the CITI training website and will inform you of the courses to be completed based on your research.
- COMMON MISTAKE: Do NOT complete any of the CITI Responsible Conduct of Research (RCR) modules. These trainings do not fulfill the human subjects research training requirements for UC Davis.
Create an IRBNet Account
All applications to the UC Davis IRB must be submitted using our electronic submission system, IRBNet. To use IRBNet, you must first register as a new user. Visit our IRBNet Information webpage for more information.
Completing a New Project on IRBNet
Create a New Project package on IRBNet
Follow along with this IRBNet Demonstration video to learn how to create a New Project.
Complete the Initial Review Application form for your New Project package
The Initial Review Application (IRA) is an online data entry form. You must log into IRBNet to access and complete the IRA.
- IRB TIP: The final page of the IRA is the Form Complete page. Under Additional Documentation, you will see a complete list of the documents that should be included in your submission to the IRB based on your answers in the IRA.
- COMMON MISTAKE: To make edits to the Initial Review Application, go to the Designer page and click the pencil icon to open the electronic data entry view of the form. Do not download the PDF and change the document.
Develop the research protocol and consent documents
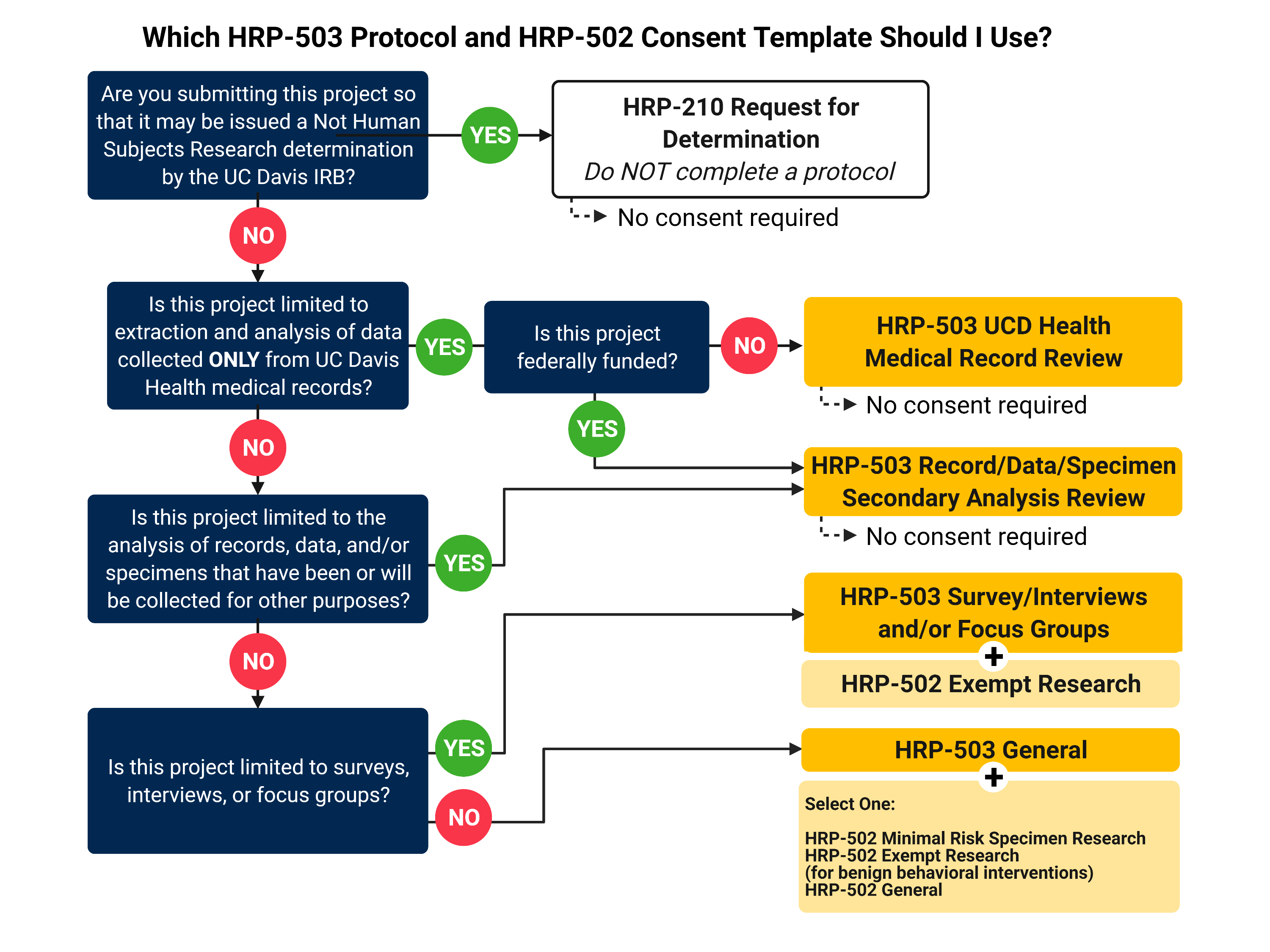 Research Protocol
Research Protocol
Each project must have one research protocol. If there is a sponsor protocol or outside researcher protocol, it can be used as the research protocol. If there is no existing research protocol, use one of our HRP-503 Protocol Templates for your project. Complete the required fields and upload the protocol in IRBNet.
- HRP-503 UCD Health Medical Record Review Template
- HRP-503 Surveys/Interviews and/or Focus Groups Review Template
- HRP-503 Record/Data/Specimen Secondary Analysis Review Template
- HRP-503 Drugs/Devices and/or Clinical Interventions
- HRP-503 General Template
Consent Document(s)
If your project involves any interaction with research subjects, consent for participation in research will be required. Select the appropriate HRP-502 template for your project.
- HRP-502 Template – Exempt Research
- HRP-502 Template for Minimal Risk Specimen Research
- HRP-502 Template for Survey/Interview Research
- HRP-502 Template – General
- IRB TIP: If the sponsor or coordinating site provided a consent document, transfer the sponsor’s language into the appropriate HRP-502 template. HRP-502 templates contain required UC Davis language.
- IRB TIP: If children are the primary subjects in research, add a sentence to the consent document indicating the word “you” refers to the child, not the parent.
Upload documents to IRBNet
Depending on the study design and research procedures, the following additional documents may be required:
- Research protocol
- Informed consent document/consent script/information sheet
- Anything intended to be seen or heard by participants
- Recruitment materials (flyers, brochures, advertisements)
- Surveys/interview/focus group questions
- Data collection instruments (assessments)
- Investigator’s Brochure or Package Insert (for a clinical trial of an investigational product)
- Ancillary review documentation
- IRB Fee Form
- IRB TIP: File names will be printed in IRB approval letters. To obtain a list of documents reviewed and manage version control, use version dates in file names when they are uploaded into IRBNet.
- COMMON MISTAKE: Do NOT submit the following: CITI training completion certificates, HIPAA authorizations, grants, conflicts of interest paperwork, abstracts, HRP-507 Short Forms, any HRP-300s Worksheets, or any HRP-400s Checklists.
Sharing Your Project and Obtaining Required Signatures
Share the project with signatories and other research personnel on IRBNet
Principal Investigators, Co-principal Investigators, and Faculty Advisors are required to have Full access to projects in IRBNet. Research coordinators, administrators, and anyone responsible for correspondence with the IRB may also benefit from having access to the study in IRBNet. Anyone with Full access to a project can share it with another user.
Follow along with this IRBNet Demonstration video to learn how to share a project.
Obtain required electronic signatures on IRBNet
All signatures must be completed using IRBNet’s Sign this Package feature. System instructions and demonstration videos are available on the IRBNet Guidance webpage.
- For all projects, the Principal Investigator.
- For all Departments, except the School of Nursing, the Department Chair.
- For the School of Nursing, the Dean.
- For principal investigators who are clinical nurses not associated with the School of Nursing, the Director for the Center for Nursing Science and Chief Nursing and Patient Care Services Officer.
- For Principal Investigators who are students, medical residents, or visiting scholars, a Faculty Advisor.
The IRBNet signatory titles below are associated with the following signatory roles:
- Principal Investigator – Principal Investigator
- Department Head – Department Chair
- Other Signatory – Dean of School of Nursing
- Advisor – Faculty Advisor
Follow along with this IRBNet Demonstration video to learn how to sign a project on IRBNet.
- IRB TIP: Provide new users with the Signatory Guide.
- COMMON MISTAKE: Designee signatures are NOT accepted. For New Projects the Co-Principal Investigator’s signature is not accepted in lieu of the Principal Investigator’s signature.
Submit the project
Once all requirements are complete, submit the application to the IRB for review. IRB Administration will screen the application for completeness and route it for review.
- COMMON MISTAKE: Don’t forget to click submit! Applications are not automatically routed for review when signatures are completed. The researcher must click the “Submit this Package” button in IRBNet to submit the application for review.
Example Submissions
The required documents to be reviewed during a New Project submission to the IRB depend on the nature of the research. Upon completion of the Initial Review Application, you will be presented with a list of required documents that need to be uploaded and submitted for review.
The following are examples of the documents required at initial review:
UC Davis Health Medical Record Review
- Initial Review Application
- HRP-503 UCD Health Medical Record Review Template
- Principal Investigator Signature
- Department Chair Signature
- Faculty Advisor Signature if applicable
Secondary Analysis of Records, Data, or Specimens
- Initial Review Application
- HRP-503 Record/Data/Specimen Review Template or a sponsor protocol (If your study has a sponsor protocol, you do not need to complete one of the HRP-503 Protocol Templates)
- Principal Investigator Signature
- Department Chair Signature
- Faculty Advisor Signature if applicable
Surveys, Interviews, or Focus Groups
- Initial Review Application
- HRP-503 Surveys/Interviews and/or Focus Group Review Template or a sponsor protocol (If your study has a sponsor protocol, you do not need to complete one of the HRP-503 Protocol Templates)
- HRP-502 Template – Exempt Research if applicable
- Surveys/Interviews/Focus Group Questions
- Recruitment Materials if applicable
- Principal Investigator Signature
- Department Chair Signature
- Faculty Advisor Signature if applicable
Clinical Trial
- Initial Review Application
- HRP-503 Drugs/Devices and/or Clinical Interventions or a sponsor protocol (If your study has a sponsor protocol, you do not need to complete one of the HRP-503 Protocol Templates)
- Recruitment Materials (flyers, brochures, advertisements)
- Screening scripts
- Data Collection Tools
- Proof Ancillary Reviews
- Disclosure of any Outside Financial Interests
- Fee Form
- Principal Investigator Signature
- Department Chair Signature
- Faculty Advisor Signature if applicable
Generic Protocol
- Initial Review Application
- HRP-503 Protocol Template or a sponsor protocol (If your study has a sponsor protocol, you do not need to complete HRP-503 Protocol Template)
- HRP-502 Template – General
- Data Collection Tools
- Recruitment Materials
- Subject-Facing Documents
- Principal Investigator Signature
- Department Chair Signature
- Faculty Advisor Signature if applicable
Additional Resources
- IRB Forms
- Frequently Asked Questions
- IRB Fees
- IRB Policies and Regulations
- UC Davis Health Research Compliance
- UC Davis Clinical Research Guidebook (Health Systems Login required)

 Institutional Review Board
Institutional Review Board