Review Process
Researchers use IRBNet to submit applications to the UC Davis IRB. Each application is evaluated by our intake team and then routed for review. Application will be assessed for review type (exempt, expedited, or full committee). IRB analysts and reviewers will use IRBNet’s Lock Feature to notify researchers of requests for revisions during the review process.
In This Section
- Intake and Screening
- Administrative Determinations
- IRB Review Types
- Requesting a Rush Review
- IRBNet Tip: The Lock Feature
Intake and Screening
All submissions to the IRB go through intake and screening. During this process, an IRB analyst checks the submission for essential elements, such as completed forms, a study protocol, required signatures, and completed human subjects protections training. If any of the elements are missing, the package will be unlocked in IRBNet and you will receive a message from an IRB analyst describing the missing elements or required action. You must respond to the requests and mark revisions complete before the IRB can complete review of the application.
Once screening is complete the study will be routed for review. Some applications will go through an administrative determination process.
Administrative Determinations
Applications for non-human research and exemption determinations are not required to meet the federally defined criteria for approval, therefore a determination can be issued using an administrative review process. Some Reports of New Information also do not require IRB approval, therefore they will be acknowledged using an administrative review process. During this process, an IRB analyst will review the information provided for required elements. If needed, the analyst will unlock the package in IRBNet and send you a message requesting revisions, justification, or clarification. You must respond to the requests and mark revisions complete before the analyst can complete the review of the information. For administrative processing purposes, the application may be assigned to an agenda date, but the date does not indicate when the research will be reviewed.
IRB Review Types
Federal regulations designate two types of IRB review:
- Expedited Review is appropriate for minimal risk research and minor modifications of greater than minimal risk research. Expedited review is conducted by a single designated review instead of a committee of reviewers. Applications routed for expedited review will be placed in the designated reviewer queue. For administrative processing purposes, the application may be assigned to an agenda date, but the date does not indicate when the research will be reviewed. These applications are reviewed based on the date they were received and the expertise of the available designated reviewers.
- Full Committee Review is used for greater than minimal risk research or studies that cannot be reviewed using the expedited procedure. There are two biomedical/clinical committees one social and behavioral committee. Full committee review takes place on specific meeting dates. Committee agendas are scheduled and sent out to reviewers about 7-10 days prior to the meeting date. Submissions will be assigned to an agenda based on this timeframe.
Regardless of the type of review, all applications are reviewed to determine if the criteria for approval are met. Review each Project Guidance topic to understand what the IRB is looking for when reviewing research.
Once the IRB has completed a review, an IRB determination letter will be issued in IRBNet.
Requesting a Rush Review
Requests for rush review or document processing will be evaluated on a case-by-case basis. Please use the Project Mail or submission comment box in IRBNet to contact IRB Administration with a rush request. Please be sure to include all of the following in your rush request message:
- The justification for the rush,
- A date by which the determination is needed, and
- Any possible impacts on subjects’ rights or welfare.
IRBNet Tip: The Lock Feature
The IRBNet lock feature serves as an indicator of when action is needed by the research team. Read each of the steps below to understand the unlock/mark revisions complete process.
Package Locked
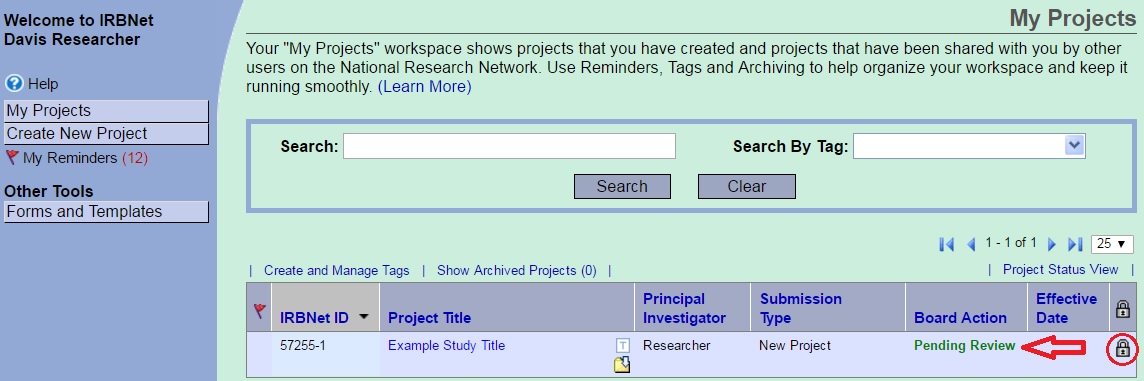
All packages are locked upon submission. This allows IRB Administration to conduct an administrative review for completeness and evaluate the proposed research without changes being made to the application. The black lock indicates the package is locked and cannot be edited.
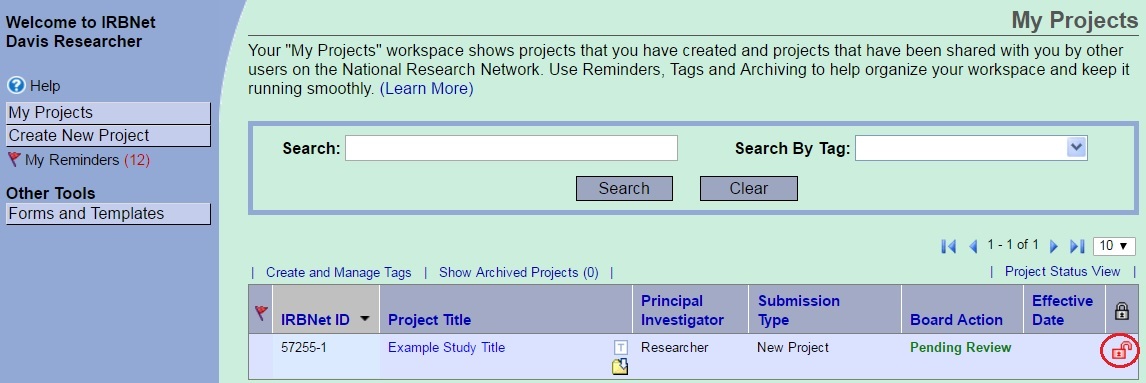
Package Unlocked
When IRB Administration requests revisions or information the package will be unlocked. The lock symbol will change from black to red. This alerts the research team that the package is available for edits and that the application requires attention. An IRB analyst will notify the research team of the action required via IRBNet Project Mail and/or IRBNet Board Documents. Review the instructions to access IRB correspondence found in Board Documents. When the package is unlocked you can edit project details, update the electronic Initial Review Application, and upload documents in the Designer. You should address all IRB requirements at this time. Once you address all items select the words “Mark Revisions Complete.”
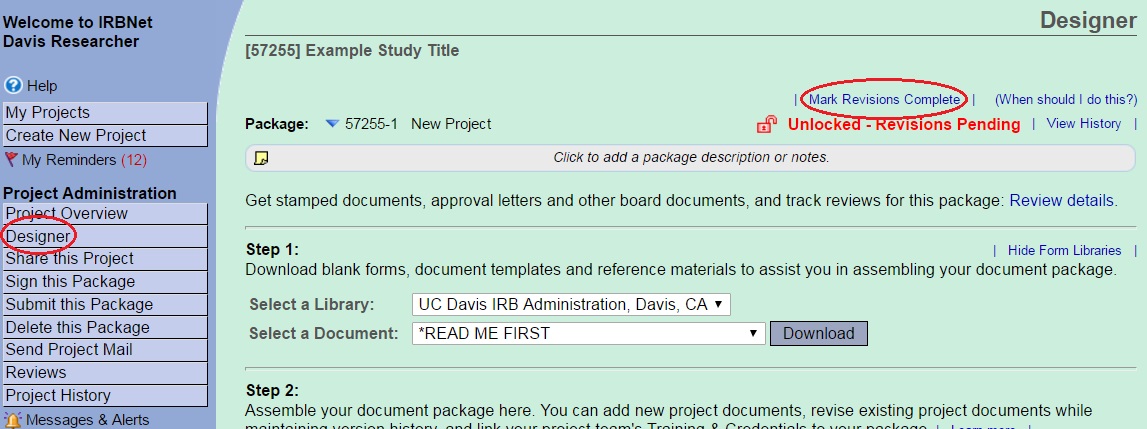
Mark Revisions Complete
When you have completed all revisions re-lock the package by going to the Designer page and selecting the words “Mark Revisions Complete” found at the top of the page. It is important that you complete this step to alert IRB Administration that you completed the requested actions and the package is ready for review. Once you mark revisions complete the package is relocked. If you have marked revisions complete and discover that you have forgotten something or need to make additional edits contact the IRB analyst reviewing your application to request that the package be unlocked. Do NOT create a new package.
After you have marked revisions complete the package will be re-locked. The application will be reviewed. If needed the package may be unlocked and additional revisions/information may be requested. The package may go through several rounds of unlock/mark revisions complete while the package’s Board Action is “Pending Review.” Once a determination has been issued the package is permanently locked.
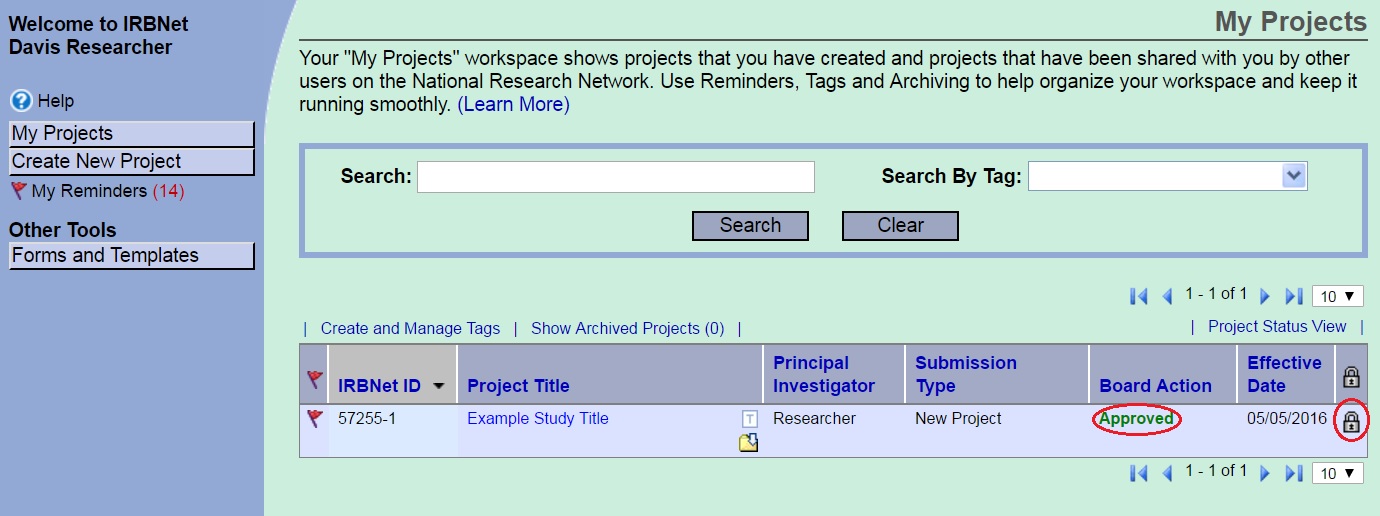
Package Locked - Board Action
Once a determination has been issued the package can no longer be unlocked. This ensures that no edits are made after the determination is issued. If you need to respond to the IRB’s determination you will need to create and submit a new package.

 Institutional Review Board
Institutional Review Board