IRB Determinations
Our electronic project management system, IRBNet, will send you notifications as the IRB processes your application. Some notifications will indicate action is needed on your part. Other notifications are sent as information only.
In This Section
- How do I know when action has been taken on my application?
- How do I access IRB correspondence and stamped documents?
- What do the different IRB actions mean?
How do I know when action has been taken on my application?
When an action is taken on your application you will receive an automatic message from IRBNet with the subject line “IRBNet Board Action.” The message will state the project details and the action taken. This is not your official correspondence from the IRB. IRB Administration will publish the official determination letter in IRBNet. When the correspondence has been published you will receive another automatic notification from the system with the subject line “IRBNet Board Documents Published” or “IRBNet Multiple Board Documents Published.” This is your alert that IRB Administration has issued the official determination. It is very important that you log into IRBNet and review the determination letter. The letter will state the determination and may also contain administrative comments and conditions that must be met related to the conduct the research.
How do I access IRB correspondence and stamped documents?
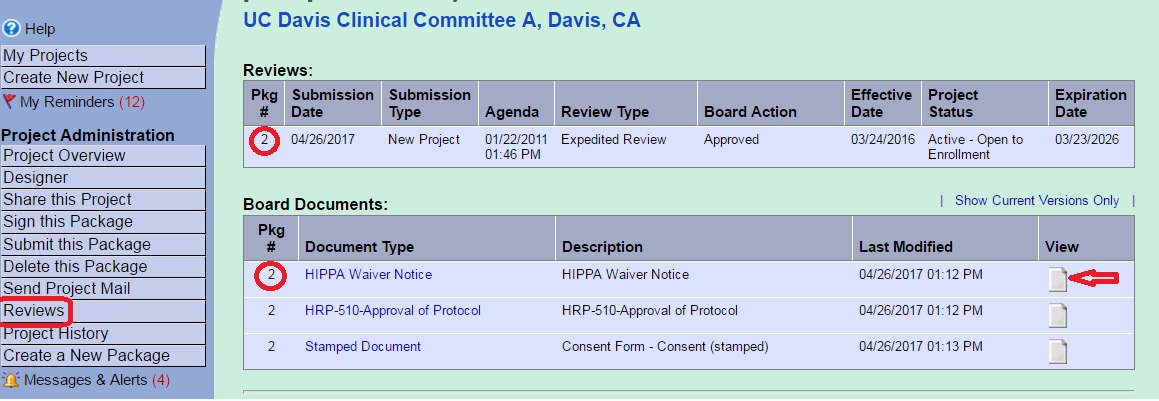
- Enter your user name and password at irbnet.org.
- Click the project title to open the protocol.
- From the left hand menu click the word “Reviews”
- The “Reviews” section lists all packages for the project. The packages are listed under the IRB Committee that conducted the review. The package number is found in the first column of the table. If you do not find the package number you are seeking scroll to the next committee section.
- There are two sections under each committee: “Reviews” and “Board Documents.” IRB correspondence, determination letters and stamped consent forms will be found in the Board Documents section. Download any Board Document by clicking the paper icon at the right.
What do the different IRB actions mean?
Not Research
The proposed activities do not meet the definition of research. Click here for more information about the definition of research.
LETTER: HRP-513-Determination Letter will be issued.
The letter is documentation of the determination. This determination applies only to the activities described in the IRB submission and does not apply should any changes be made. If changes are being considered and there are questions about whether IRB review is needed, please submit a modification to the IRB for another determination.
Research – Not Human Subject Research (NHSR)
The proposed research activities do not involve human subjects. Click here for more information about the definition of human subjects.
LETTER: HRP-513-Determination Letter will be issued.
The letter is documentation of the determination. This determination applies only to the activities described in the IRB submission and does not apply should any changes be made. If changes are being considered and there are questions about whether IRB review is needed, please submit a modification to the IRB for another determination.
Exempt
The human subjects research activities meet the criteria for an exemption. Click here for more information about exempt research.
LETTER: HRP-513-Determination Letter will be issued.
The letter is documentation of the determination. This determination applies only to the activities described in the IRB submission and does not apply should any changes be made. If changes are being considered and there are questions about whether IRB review is needed, please submit a modification to the IRB for another determination.
Research – Not Engaged
UC Davis is not engaged such that a particular non-exempt human subjects research project or activity is not subject to UC Davis’s IRB oversight. Click here for more information about engagement in research.
LETTER: HRP-513-Determination Letter will be issued.
The letter is documentation of the determination. This determination applies only to the activities described in the IRB submission and does not apply should any changes be made. If changes are being considered and there are questions about whether IRB review is needed, please submit a modification to the IRB for another determination.
Forwarded
This administrative action is taken when a submission is routed to an IRB committee for review. No correspondence is issued by the IRB at this time. The investigator should await further correspondence from the IRB.
Acknowledged
The IRB acknowledges the information provided by the investigator. This action is taken when IRB approval is not required.
LETTER: HRP-519 Review of Information Item will be issued.
The letter may contain administrative comments or conditions that must be met related to the conduct of the research.
Closed
The investigator has reported that all human subjects research activities and all analysis of identifiable data are complete OR the investigator has failed to submit an application for continuing review and IRB approval of the study has lapsed; all human subject research activities, including analysis of identifiable data must stop. The project is closed with the IRB. Investigators should ensure all required research records are retained for the appropriate length of time.
LETTER: HRP-511 Acknowledgement of Research Closure will be issued.
Approved
The ethical criteria are satisfied for the conduct of research. Research can begin when all other institutional approvals have been obtained. The IRB may provide administrative comments about some aspect of the project or its conduct outside of criteria for approval.
HRP-510 Approval of Protocol or HRP-510 Approval of Protocol (Abridged) will be issued.
What is the difference between the two HRP 510s?
- HRP-510 Approval of Protocol: This is the IRB’s correspondence documenting approval of the application. As of April 2017 this letter this letter contains detailed, study specific information about what has been approved by the IRB. Some of the determinations found in this letter include the requirements for consent process and documentation, or a waiver or alteration thereof, the approved number of subjects that may be enrolled, the date that IRB approval expires, and any administrative comments or conditions that must be met related to the conduct of the research. The IRB will issue this letter at initial approval and anytime modifications to an existing study alter determinations outlined in the letter. Click here to view a guide to the contents of HRP-510 Approval of Protocol.
- HRP-510 Approval of Protocol (Abridged): This is the IRB’s correspondence documenting approval of an application for Continuing Review or Modification when changes made do not alter the determinations outlined in HRP-510 Approval of Protocol. The letter documents the IRB’s approval of the application. The letter may include administrative comments or conditions that must be met in the conduct of the research.
Modifications Required
The IRB requires modifications in order to approve the research. Research cannot commence until a final approval is received.
LETTER: HRP-512-Modifications Required to Secure Approval will be issued.
The required revisions will be described in HRP-512 Modifications Required to Secure Approval (also referred to as the letter of action). The investigator must submit a response via IRBNet, and provide a point-by-point response memo. If the investigator makes all revisions to study documents exactly as described in the letter of action, IRB approval may be issued by a designated reviewer and the response will not need to go to a convened meeting. If the revisions submitted in the response are not exactly as described in the letter of action, or if the response contains revisions in addition to those requested, the response will be assigned to an agenda for full committee review. Click here for step-by-step instructions on how to submit a response.
Deferred
The IRB cannot approve the research as submitted and describes reasons or modifications that might make the research approvable; the IRB requests additional information from the researcher.
LETTER: HRP-516-Deferral of Protocol will be issued.
HRP-516 Deferral of Protocol will outline the specific criteria for approval that have not been met and describe the additional information, justification, or changes needed before approval can be reconsidered. The investigator must submit a response via IRBNet, and provide a point-by-point response memo. The investigator’s response will be reviewed by the full committee at a convened meeting. Click here for step-by-step instructions on how to submit a response.
Not Approved
The IRB cannot approve the research as submitted and cannot describe modifications that might make the research approvable. If the IRB disapproves the Human Research, the letter will include a statement of the reasons for disapproval and give you an opportunity to respond in writing.
The committee has found that the study design, research risks and benefits, consent process, and/or documentation of consent are inappropriate or inadequately described, such that the committee cannot specify changes necessary to meet the criteria for approval. This determination is made very rarely. The investigator’s response to the committee will typically require major revision to the study’s procedures, inclusion/exclusion criteria, or aims and will need to be reviewed by the full committee.
Suspended or Terminated
The IRB has determined that current approved research does not qualify for Approval or Modifications Required. A letter will be issued outlining the specific research activities that have been suspended or terminated.
Tabled
The IRB did not make a determination about your application. You will receive correspondence with an explanation of the reason for the action and, if necessary, a request for more information.
HIPAA Waiver
It is always preferred to obtain authorization to use an individual’s PHI; however, when certain conditions are met the IRB may issue a waiver of the requirement for authorization. For more information about HIPAA click here.
When the IRB approves a HIPAA waiver, a HIPAA Waiver Notice will be issued.
This notice is documentation of the IRB’s determination related to the disclosure or use of protected health information. The type of waiver and the requirements for compliance with HIPAA are described in the letter. The investigator must ensure compliance with the conditions described in the letter. As of April 2017, the HIPAA Waiver Notice is issued instead of Form R and Form W.
Stamped Documents
The UC Davis IRB stamps consent documents only at initial approval and when a modified version is approved. Please note that you must not use a modified informed consent document until it has been approved and stamped with the new approval date.

Click here to view our guidance document on stamping.
Stamped consent forms will be published in Reviews section of IRBNet under Board Documents.
Please note that the UC Davis IRB Administration uses an automated stamping process. This process allows for faster stamping of consent documents once they have been approved and reduces the possibility of errors in protocol numbers and approval dates found in the stamp. The stamping process automatically converts documents from Word to PDF and applies a stamp in the lower right corner of each page.
To ensure the stamp does not cover text on the consent document, it is important the you submit consent documents that allow enough space for the stamp. To avoid problems with the stamping process please follow these guidelines:
- Set the bottom margin at 1.3”
- Save all pages in portrait orientation (not landscape)
- Submit consent documents as PDF files
Please review HRP-052 for information on signatures on IRB correspondence.

 Institutional Review Board
Institutional Review Board