Single IRB and Reliances
This page is designed to provide an overview of the IRB reliance agreement process at UC Davis. If you need additional information, please contact the Reliance Team for assistance.
In This Section
- Helpful Definitions
- Regulatory Requirements
- UC Davis Reliance Policy
- UC Davis to Act as the Reviewing IRB for Participating Sites
- UC Davis as a Participating Site Relying on an External IRB
- Additional Resources
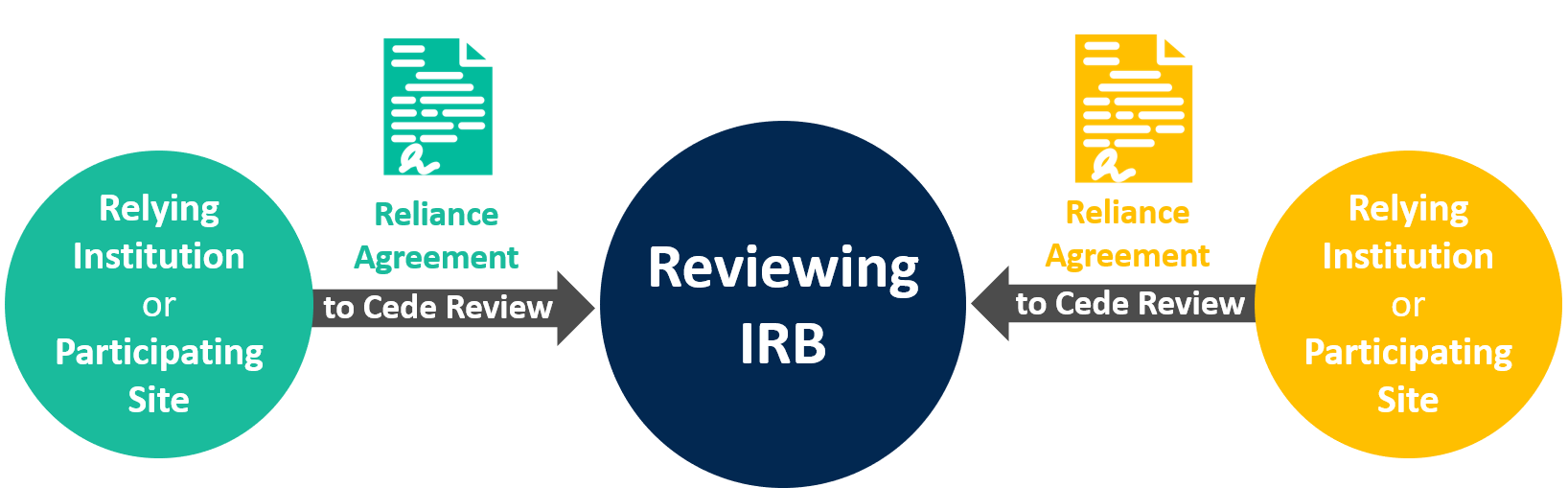
Helpful Definitions
Reviewing IRB
An Institutional Review Board responsible for overseeing all sites participating in a multi-site study. Sometimes also referred to as the IRB of record,single IRB (sIRB), or central IRB (cIRB).
Relying Institution or Participating Site
A relying institution or participating site is an institution or site that has entered into an IRB reliance agreement with a reviewing IRB to carry out the relying institution/participating site’s IRB review. A relying institution or participating site may or may not have its own IRB. If it does, the relying institution or participating site’s IRB is called the relying IRB.
Reliance Agreement
A formal, written document that provides a mechanism for an institution engaged in non-exempt human subjects research to delegate IRB review to an IRB affiliated with another institution or an independent IRB. Sometimes also referred to as an IRB Authorization Agreement (IAA).
External IRB
An IRB unaffiliated with the institution or site being discussed. For example, any IRB that is not the UC Davis IRB is considered an external IRB to UC Davis.
Non-Exempt Human Subjects Research
A project that meets the federal definition of human subjects research and requires IRB review at the Expedited or Full Board level.
Reliance Fees
Fees may apply for single IRB review. In general, relying institutions or participating sites relying on the UC Davis IRB will need to pay a fee for review. Industry-sponsored studies for which UC Davis is a participating site will also need to pay a fee for review. Click here for more information.
Cede Review
The act of transferring responsibility of IRB review to an IRB at another institution or an independent IRB.
IRB of Record
See definition of Reviewing IRB above.
Central IRB (cIRB)
See definition of Reviewing IRB above.
Single IRB (sIRB)
See definition of Reviewing IRB above.
Relying IRB
See definition of Relying Institution or Participating Site above.
IRB Authorization Agreement (IAA)
See definition of Reliance Agreement above.
Individual Investigator Agreement (IAA)
A formal written agreement between an institution conducting research and an independent investigator who is collaborating on the research where the institution agrees to extend its Federalwide Assurance (FWA) to the independent investigator if (s)he agrees to fulfill specified expectations and responsibilities.
Regulatory Requirements
NIH Policy
Effective January 25, 2018, the NIH requires the use of a single IRB (sIRB) for the review of NIH-funded multi-site studies where each site will conduct the same protocol involving non-exempt human subjects research, whether supported through grants, cooperative agreements, contracts, or the NIH Intramural Research Program. This policy applies to sites in the United States only.
Revised Common Rule
The Common Rule is a federal policy regarding Human Subjects Protection that applies to 20 Federal departments and agencies. Under the revised Common Rule, most U.S. government-funded cooperative studies that meet the criteria for non-exempt human subjects research, and involve more than one site, will require sIRB review. This requirement went into effect January 20, 2020.
UC Davis Reliance Policy
When UC Davis investigators are conducting collaborative, non-exempt human subjects research (i.e., the human subjects research requires expedited or full board review), it may be possible to establish an IRB Authorization Agreement (IAA) allowing one institution to rely on IRB review conducted by another IRB.
Activities that do not constitute human subjects research or are determined to be exempt are ineligible for a reliance agreement. Instead, the UC Davis IRB will perform IRB review, if required. In addition, UC Davis does not routinely execute reliance agreements with international institutions.
UC Davis to Act as the Reviewing IRB for Participating Sites
The following information applies when relying institutions or participating sites cede IRB review to the UC Davis IRB.
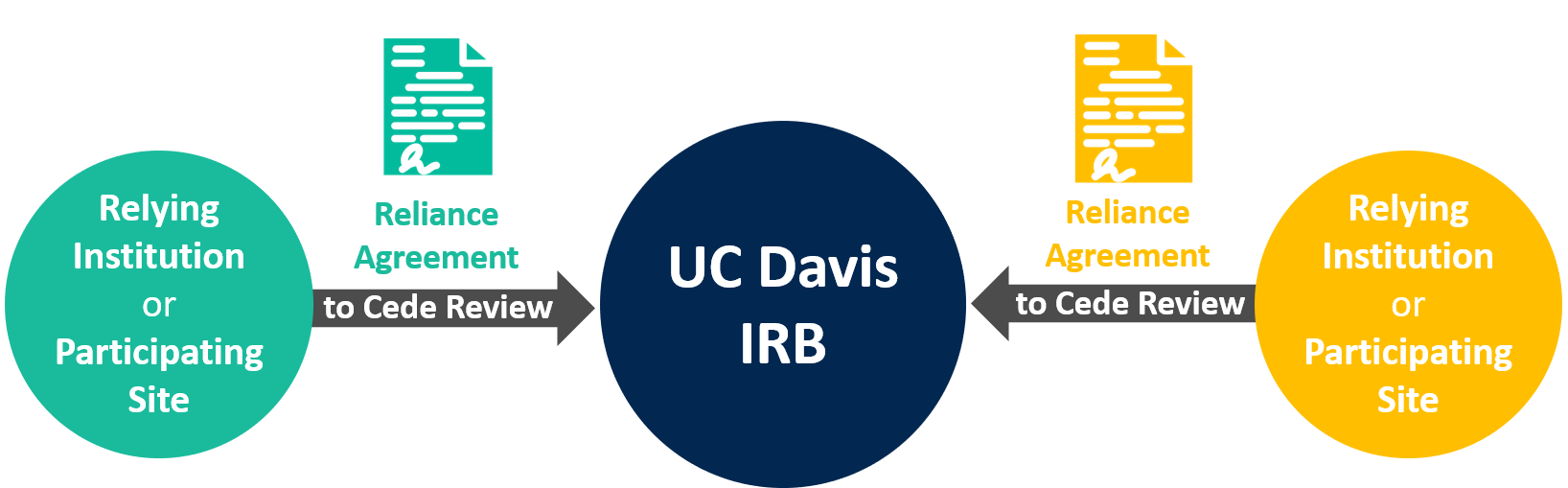
How to Initiate a Reliance Agreement when UC Davis is the Reviewing IRB
Prior to submission to the IRB, send an email to the Reliance Team to request consultation. The Reliance Team will:
- Determine reliance eligibility
- Provide guidance for how to submit to the IRB
- Obtain IRB Authorization Agreements, when applicable
Including the following information in the email:
- Study title
- Funding source
- Status of funding (funded or pending funding)
- Name of the UCD PI
- List of institutions that are expected to rely on UC Davis IRB and contact information for the PI and IRB
- Protocol or study summary
- Description of the activities to be conducted by the collaborating researchers
Training Video
Table of Contents
Click on any of the hyperlinks below to jump directly to the part of the training video above where this topic is discussed.
- Terminology
- Initial Submission
- IRBNet Demonstration: Completing the Study-Wide Package
- IRBNet Demonstration: Completing the UC Davis Package
- Post-Approval Submissions Overview
- Modifications
- Reportable Events
- Continuing Review
- Closures
- Fee Form
- Questions
Responsibilities of the UC Davis PI when UC Davis is the Reviewing IRB
In addition to the normal responsibilities, the UC Davis PI is responsible for:
- Communicating to relying investigators that they must submit reportable events to their local IRB following local IRB reporting requirements, and
- Actively communicating with study investigators at the relying campus sites to ensure post-approval activities take place, including submission of modifications and continuing review for the relying sites to UC Davis IRB.
HIPAA when UC Davis is the Reviewing IRB
If a waiver of HIPAA Authorization is requested, it may be issued by the UC Davis IRB or the relying IRB. This will be communicated via project mail and in determination letters on IRBNet.
UC Davis as a Participating Site Relying on an External IRB
The following information applies when the UC Davis IRB cedes IRB review to an external IRB.
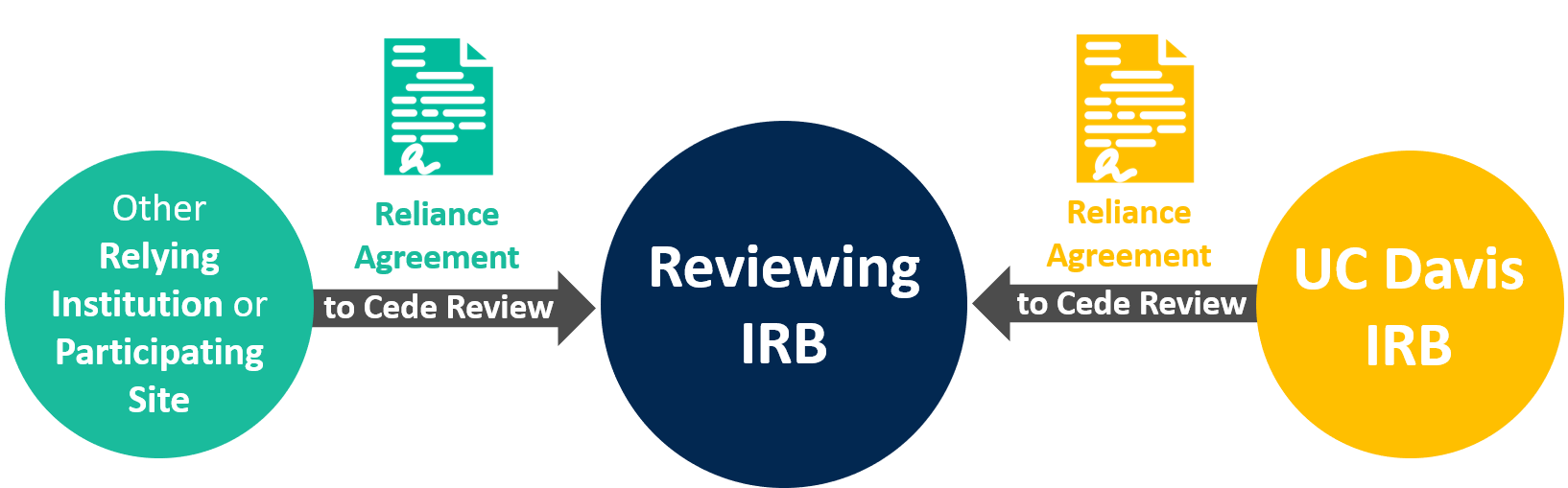
How to Initiate a Reliance Agreement when Relying on an External IRB
If the study qualifies for a reliance agreement, submit the following to the IRB via IRBNet:
- Initial Approval Document from the proposed IRB of Record
- Initial Review Application
- Protocol
- Consent Documents
- UCD Required Consent Language
- UC Davis Ancillary Committee Approvals/Determinations
- Reliance Fee Form (use the IRB Fee Form Calculator to determine applicability)
- Recruitment Materials
Training Video
Table of Contents
Click on any of the hyperlinks below to jump directly to the part of the training video above where this topic is discussed.
- Overview and Terminology
- Reliance Agreement Process
- Initial Submission
- Initial Review Application in IRBNet
- Review Details in IRBNet
- Question Break
- Post-Approval Submissions Overview
- After Approval, What Do I Need to Submit to the UC Davis IRB?
- Modifications to Local Context
- Reportable Event Determinations to Be Submitted to the UC Davis IRB
- Study Closure
- Questions
Responsibilities of the UC Davis PI when Relying on an External IRB
In general, when relying on an external IRB, the UC Davis PI must follow the reviewing IRB’s policies. All modifications, continuing review progress reports, and other required submissions should be reported to the IRB of record.
The UC Davis IRB should receive a very limited number of submissions when it is not the reviewing IRB. The following must be submitted to the UC Davis IRB in addition to any reporting required by the IRB of record:
- Modification when there is a change in PI or Co-PI
- Other Reportable Event when the IRB of record has made any of the following determinations:
- Suspension
- Serious Non-Compliance
- Continuing Non-Compliance
- Unanticipated Problems Involving Risk to Subjects or Others
- Closure when the study is closed permanently at UC Davis
In addition, if there is a change in research that impacts UC Davis local context approval, please contact the UCD Reliance Team. These changes may require additional reporting to the UCD IRB. Examples include, but are not limited to:
- Addition of Prisoners as research subjects.
- New Exception from Informed Consent Requirements for Emergency Research (EFIC)
- New or change to a Waiver of Consent or Waiver of HIPAA Authorization
- Change to UCD required consent language
The UC Davis PI is required to conduct the research in compliance with pertinent federal regulations, state law, University of California policies, and UC Davis policies.
HIPAA when Relying on an External IRB
UC Davis requires the use of a stand-alone document for obtaining HIPAA authorizations. As such, HIPAA authorization language cannot be embedded within the consent documents. Changes to the UC Davis approved HIPAA Authorization will require negotiations with the Compliance Department.
If you will be obtaining a signed HIPAA authorization from your subjects, you must use the UC Davis approved HIPAA Authorization found on the IRB Forms webpage. If you will need a waiver of HIPAA authorization for all or part of your study (e.g., recruitment), a request will need to be made to the IRB of record.

 Institutional Review Board
Institutional Review Board