IRB News November 2023
Want to stay informed of the latest UC Davis Institutional Review Board news? Subscribe to the IRB listserv.
In This Edition
- Employment Opportunity
- Holiday Closure
- Don’t Let Your IRB Approval Expire!
- New IRB Roster and 2024 Committee Meeting Dates
- Training Corner
- Weekly Virtual IRB Office Hours
Employment Opportunity
Do you dream of IRB trainings? Can you navigate IRBNet with your ears closed? Is your reading comprehension sharp enough that you caught the mistake in the last sentence? If you answered yes to these questions, come join us at the IRB!
IRB Administration is recruiting a Research Compliance Analyst 3 to support our Systems and Education team. The last day to apply is November 10!
Research Compliance Analyst 3
Requisition Number: 60815
Payroll Title: RSCH CMPLNC ANL 3
Percentage of Time: 60% Variable
LINK to posting
Holiday Closure
IRB Administration will be operating with limited staff Monday, December 25, 2023 through Monday, January 1, 2023. We will re-open Tuesday, January 2, 2024. Additionally, we historically see a large influx of submissions in January. As such, you may experience longer than typical processing times for IRB submissions.
If you require a determination from the IRB during the winter holidays or early 2024, please plan ahead and submit early. In addition, make sure to include any relevant deadlines for research activities, so that reviews may be prioritized appropriately.
During the holiday closure, please call (530) 219-7951 or email [email protected]for any urgent issues.
Don’t Let Your IRB Approval Expire!
In response to an increase in the number of studies missing the administrative due date for continuing review of approved research, we have asked IRBNet to reconfigure the settings for automated continuing review reminders. All studies requiring continuing review will now be sent an automated reminder to submit a Continuing Review package, regardless of whether or not there is a package (e.g., reportable event or modification) pending review. If you receive an automated reminder after you have submitted a continuing review package, then you may ignore this message.
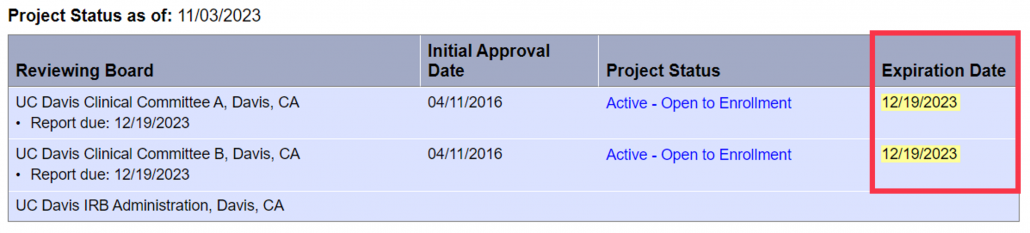
Nonetheless, we encourage researchers not to rely on automated messages. You may look up the expiration date for any study by navigating to the “Project Overview” for your study (see below). The administrative due date to submit a Continuing Review is 45 days prior to expiration. For example, the administrative due date for the study below is 11/04/2023.
New IRB Roster and 2024 Committee Dates
In addition to our 2024 committee meeting dates, a new roster of IRB reviewers has been posted on our About IRB webpage. This document is oftentimes requested by sponsors of research regulated by the FDA.
Training Corner
Diversity, Equity, and Inclusion in Clinical Research Recruitment
Date: Thursday, November 30, 2023
Time: 10:00 AM – 12:00 PM
Description: This course explores the importance of diversity, equity, and inclusion in clinical research recruitment. Participants will learn practical skills to engage diverse communities, develop inclusive outreach strategies, and foster a more representative research environment.
LINK to enroll
Weekly Virtual IRB Office Hours
The IRB holds weekly drop-in office hours every Friday from 12:00 PM – 1:00 PM. To join virtual office hours at the following times, please use this LINK:
- November 10 | No Office Hours (UC Holiday)
- November 17 | 12:00 PM–1:00 PM
- November 24 | No Office Hours (UC Holiday)

 Institutional Review Board
Institutional Review Board